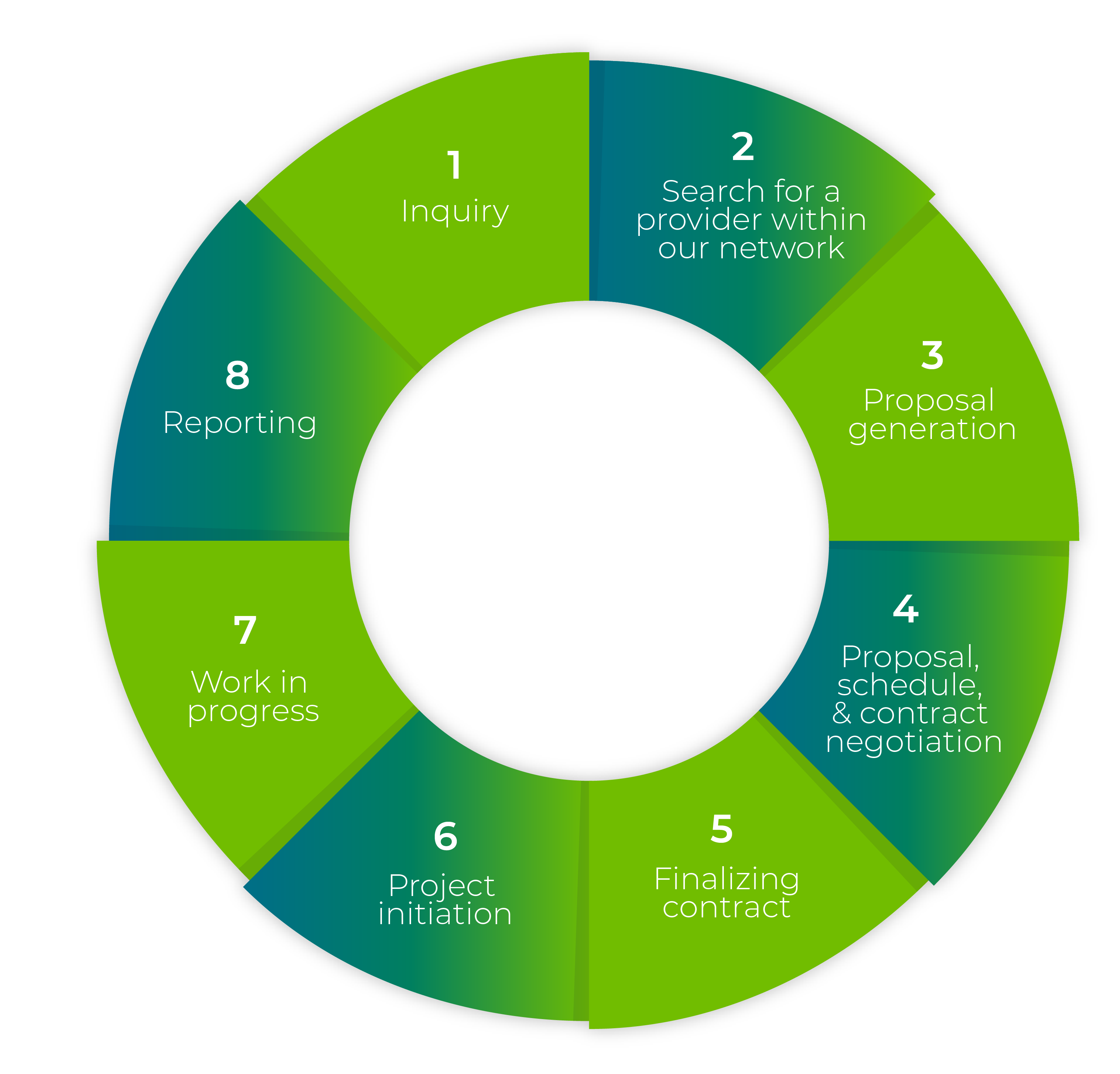
Customized Solutions
Adapted to Changing Needs
Connect with the Right CRO/CDMO Suppliers Faster, Secure Better Prices, and Achieve Superior Outcomes with Our Support.
Unlock the Full Potential of Your Research with BioPulse Consulting
At BioPulse Consulting, we specialize in connecting biopharma and biotech companies and start-ups with the best CROS and CDMOS tailored to your specific needs. Our services go beyond just matching; we act as your dedicated CRO consultant, ensuring that you receive the highest quality services at the most competitive pricing.
Unlike other platforms, we provide personalized support from industry experts, ensuring you’re never left to navigate the process alone. We stay with you throughout the entire project, offering continuous guidance and support. Experience the BioPulse difference as we manage the complexities of your research, saving you valuable time, money, and resources.
Why BioPulse Consulting?
Here are a few of the many reasons to use BioPulse Consulting if you are starting a new study and searching for the right CRO/CDMO.

Comprehensive CRO Consulting Services
We offer a full range of CRO consulting services, from identifying the right partners to managing the entire lifecycle of your projects. Our expertise ensures that you get the best deals. and the most suitable partners for your unique requirements.

Unmatched Value and Quality
BioPulse is known as a premier value provider in the industry. We maintain strong partnerships with top-tier CROs and CDMOs, guaranteeing that our clients receive unbeatable pricing and non-negotiable quality. Our motto is simple: “If we cannot find it, it doesn’t exist.”

Experienced Leadership with a Proven Track Record
With over 20 years of experience in regulatory environments, our leadership team brings a deep scientific background to the table. We know the right questions to ask and the critical aspects to consider, ensuring that every project meets the highest standards.

Personalized Support & Lasting Relationships
Unlike our competitors, who offer DIY platforms, BioPulse provides personalized support from real people. This hands-on approach helps you achieve optimal pricing and secure better deals. Building a lasting relationship with our team means you can engage and re-engage us whenever you start a new study, ensuring you always get the best match and price based on your individual requirements.
Service Offerings
At BioPulse Consulting, our strength lies in our expansive network of top-tier CROs, CDMOs, and expert consultants. We connect you with the right partners and offer a comprehensive suite of services tailored to your specific needs. Whether you require specialized toxicology consulting, custom analytical method development, or clinical trial execution, our offerings are designed to support your research from start to finish.

Premiere Liaison & Program Management
Our extensive network of providers and comprehensive program management services ensure seamless project execution from start to finish.

Comprehensive CRO Outsourcing
We simplify the process of CRO outsourcing by managing negotiations, contracts, and scheduling, saving you valuable time and effort. Our clients often receive preferential support, further enhancing the value we provide.

Specialized Toxicology Consulting
Whatever your toxicology needs, whether the route of administration, species, or drug type (small molecule or biologic), our CRO partners have you covered. We offer specialized toxicology consulting services, ensuring that all aspects of your studies are handled with the utmost care and expertise.

Custom Analytical Method Development
Our CRO partners provide regulated, customized analytical method development, transfer, and validation using state-of-the-art analytical tools such as HPLC (with UV, CAD, RI, ELSD, FLD, and conductivity detectors), UPLC, lon Chromatograph, LC-MS/MS, GC, ICP-MS, and GC- MS.

Clinical Trial Execution & Patient Recruitment
Our Clinical CRO partners bring a wealth of expertise in conducting clinical trials and patient recruitment across multiple therapeutic areas.

Biomarkers & Bioanalysis Support
We understand that bioanalytical methods can be challenging, and finding the right CRO can be equally difficult. Our CRO partners provide exceptional support for method development, validation, and analysis for both small molecules and biologics, utilizing state-of-the-art instrumentation.

Regulatory & Scientific Consulting
Our network of consultants is vast, encompassing both regulatory and scientific experts to support your unique needs.
Benefits

Competitive Advantage
By handling the tedious process of sourcing and negotiating with CROs/CDMOs, BioPulse allows you to focus on your core research activities. This saves you time and ensures that your projects move forward without unnecessary delays.

Cost Savings and Risk Management
We secure preferential pricing for our clients, leading to significant cost savings. Our hands-on approach to logistics and oversight management ensures any issues are swiftly resolved, reducing risks and keeping your project on track.

Comprehensive Consulting Support
We offer full-spectrum consulting, from referrals to project management. Our consulting support covers every stage of your project, ensuring all your needs are met with precision. Whether it’s pharmaceutical consulting or broader project support, we’re with you from start to finish.
BioPulsers
Meet the dedicated professionals who drive our success, bringing a wealth of expertise and passion to every project we undertake

Kyle Abuarjah
Founder & CEO

Dr. Suliman Al-Fayoumi
Scientific Advisor,
Clinical Pharmacology

Dr. Philip Oldfield
Scientific Advisor-Bioanalysis

Kyle Abuarjah
FOUNDER & CEO
Kyle Abuarjah has over 20 years of experience in the CRO industry in the Large Molecule arena. Kyle started BioPulse Consulting to serve an unmet need and provide a cost effective outsourcing model for both CROs/CDMOs and Biopharmaceutical companies. Prior to BioPulse, Kyle provided Business Development leadership for Primera Analytical Solutions, Alliance Pharma, CMIC, Charles River Laboratories, SGS Life Sciences, and Quest Diagnostics. In addition, Kyle provided leadership in building the Large Molecule Bioanalytical lab operation at CMIC (formerly JCL Bioassay USA). Kyle began his career on the bench as a Medical Laboratory Technologist in diagnostics. Then, joined the CRO industry and worked in different roles in Immunoassay bioanalysis including Project Management, Business Development, and as a Principal Investigator. Kyle received his BSc in Clinical Laboratory Sciences from the University of Texas Medical Branch at Galveston and received his Masters of Business Administration (MBA) from Athabasca University. Kyle is certified by the American Society of Clinical Pathology (ASCP) and a member of the American Association of Pharmaceutical Scientists (AAPS). In his free time, when there is a free time, Kyle spends his time with Prada (Ms. Parudi) the kitty office manager.

Dr. Suliman Al-Fayoumi
SCIENTIFIC ADVISOR- CLINICAL PHARMACOLOG
Dr. Suliman Al-Fayoumi is currently a senior Consultant with Nuventra supporting several small Biotech companies primarily based in USA. Prior to taking-up consulting in 2017 then joining Allucent in 2021, Dr. Al-Fayoumi served as Vice President of Translational Sciences and Clinical Pharmacology at Cell Therapeutics Biopharma Inc., a niche hematology-oncology company based in Seattle, WA between 2013-2017. Prior to joining CTI Biopharma, Dr. Al-Fayoumi served as Director of Preclinical and Clinical Pharmacology and Interim Head of Research at Acucela Inc., a niche Ophthalmology company based in Seattle, WA between 2010-2013. Prior to joining Acucela, Dr. Al-Fayoumi served as Associate Director of Global PK/PD within the DMPK Department at Novartis Pharmaceuticals (East Hanover, NJ), where he supported the Cardiovascular & Metabolism (CVM) Franchise between 2006-2010. Prior to Joining Novartis Pharmaceuticals, Dr. Al-Fayoumi served as Senior Clinical Pharmacology Reviewer at US FDA between 1999-2006 supporting GI, anticoagulants, anesthetics, addiction and dermatology Drug Product Divisions. While at FDA, Dr. Al-Fayoumi was the primary clinical pharmacology reviewer for several high profile drug applications such as Nexium, Prilosec OTC, Prevacid, Oxycontin, Ximelagatran, Asacol, Remicade, Lovenox, Innohep, Exjade, Propulsid, Etreva, Fabrazyme, Zegerid, Protonix, Lotronex and Zelnorm. Dr. Al-Fayoumi received several awards for outstanding support of and significant contributions to the achievement of excellence in FDA drug safety initiatives related to optimal dosing and lowest effective dose including CDER’s Frances O. Kelsey Drug Safety Excellence Award. Dr. Al-Fayoumi has a B.S. degree in Pharmacy from Jordan University of Science and Technology. Dr. Al-Fayoumi received a Ph.D. in Pharmacokinetics from the University of Florida (Gainesville, FL) in 1998, and MBA degree in International Business from Fairleigh Dickinson University (Madison, NJ) in 2009. Dr. Al-Fayoumi is a member of the American Diabetes Association (ADA), the American College of Clinical Pharmacology (ACCP), the American Society of Clinical Pharmacology and Therapeutics (ASCPT), the American Association of Pharmaceutical Sciences (AAPS), European Hematology Association (EHA), and The American Society of Clinical Oncology (ASCO).

Dr. Philip Oldfield
Scientific Advisor-Bioanalysis
Dr. Philip Oldfield started his scientific career in 1974 in the Clinical Biochemistry Department at the Royal Postgraduate Medical School, Hammersmith Hospital London, England. Since then he has obtained three degrees; including a D.Phil obtained in 1982. The subject of his research thesis was “Proteolytic Control and Rheumatoid Disease”. In 1986 he was given the Baker Award for his work on Digoxin-Like-Immunoreactive-Substances. Dr Oldfield has had over 25 years experience in service to the pharmaceutical and biopharmaceutical industry specializing in LBA techniques and Good Laboratory Practice. Dr Oldfield is an Associate Member of the Royal College of Pathologists, a Fellow of the Royal Society of Chemistry, and a Member of the American Association of Pharmaceutical Scientists (currently the Past Chair of the Ligand Binding Assay Bioanalytical Focus Group). In conjunction with BioPulse, Dr Oldfield operates Philip Oldfield Bioanalytical Consulting where he provides strategic bioanalytical consulting with a commitment to quality focusing on biological and oligonucleotide therapeutics. Prior to that, Dr Oldfield was Scientific Director at Charles River Laboratories where he managed the scientific and technical operation for the Immunoassay group. In addition, he likes to maintain a link with the local universities, and has been involved in supervising postgraduate students, as well the occasional lecture. He is currently involved as a committee member to organize symposia and workshops as part of the Drug Development Program Course at the University of McGill.
Testimonials
What Our Partners Say About Us
Discover how BioPulse Consulting has made a difference for our clients. From start-ups to established biopharma companies, our commitment to excellence and personalized support has led to lasting partnerships and successful outcomes.
How We Compare with Our Competitors
Our clients consistently report higher satisfaction and better outcomes.
Ready to see how we can transform
your research projects?
Get a personalized consultation and discover how BioPulse Consulting simplifies finding the right CRO/CDMO suppliers,
securing the best prices, and managing your projects from start to finish. Let us show you the
difference our expert team can make in your research journey.